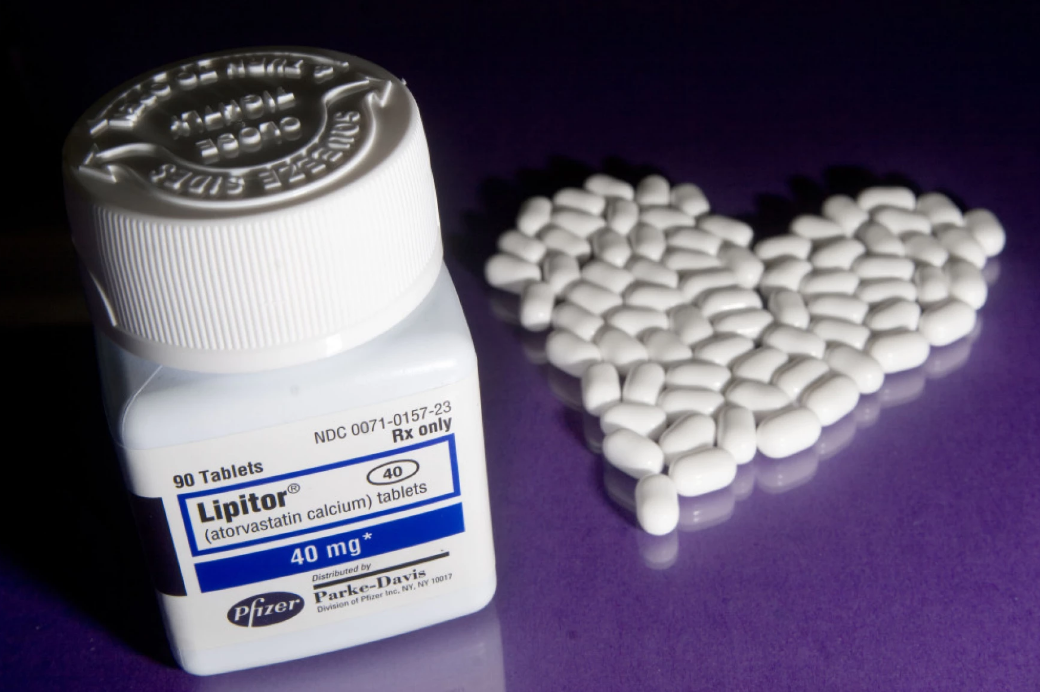
CHARLOTTE — There’s a recall for thousands of bottles of cholesterol medication. The FDA said Ascend Laboratories is pulling atorvastatin calcium tablets, used to treat high cholesterol. They said the pills may not dissolve properly after you take them.
The recall applies to the 10, 20, 40 and 80 milligram tablets in the 90-count bottles. This is a class two recall, meaning the effects are temporary or medically reversable.
Which Medicine Is Being Recalled?
The recall affects atorvastatin calcium tablets — the generic version of Lipitor, widely used to manage cholesterol and prevent heart disease. These tablets are being pulled due to issues with how they dissolve after being swallowed, which may affect how well the medicine works.
Details of the Recalled Products:
- Brand: Ascend Laboratories
- Dosages affected: 10 mg, 20 mg, 40 mg, and 80 mg
- Pack size: 90-count bottles
- Type of recall: Class II (meaning temporary or reversible health effects)
Why is This Important?
According to the FDA, the tablets may not dissolve properly in the body. This could reduce the effectiveness of the drug, meaning it may not control cholesterol levels as expected.

While this issue isn’t likely to cause long-term harm, it could have short-term health impacts, especially for people with serious heart-related conditions.
What is a Class II Recall?
A Class II recall is used when a product may cause temporary or medically reversible health problems, or where the chance of serious harm is very low. In this case, patients taking the recalled atorvastatin may not experience serious side effects, but their cholesterol may not be managed properly.
What Should You Do If You Have This Medicine?
If you’re currently taking atorvastatin calcium from Ascend Laboratories:
- Check the label for the brand and dosage. If it matches the recall list, contact your pharmacist or doctor.
- Do not stop taking the medicine without speaking to a healthcare provider. Stopping suddenly may increase your cholesterol or risk of heart problems.
- Pharmacists may replace the medicine with a different batch or brand.
Common Signs the Drug Isn’t Working Well:
If the medication isn’t dissolving properly, some people may notice:
- No improvement in cholesterol levels
- Unusual fatigue or chest discomfort (rare but possible)
- Lack of expected results from regular health checkups
If you notice anything unusual, talk to your doctor.
How Serious is This Recall?
This recall is precautionary. The FDA and Ascend Laboratories are acting early to avoid potential problems. There are no reports of injury or illness at this time. But it’s a reminder that drug quality matters – and how a tablet breaks down in your body is just as important as its ingredients.
Ascend Laboratories’ recall of atorvastatin calcium tablets highlights a common but important issue in medication safety — how a tablet behaves after it’s swallowed. If you or a loved one take this medication, stay informed, check your bottle, and contact a healthcare provider for guidance. Though the risk is low, it’s always better to act early when it comes to your health.
FAQs
Why was atorvastatin recalled?
Ascend Laboratories recalled it because the tablets may not dissolve properly, which could affect how well the drug works.
Which atorvastatin doses are included in the recall?
The recall includes 10 mg, 20 mg, 40 mg, and 80 mg tablets in 90-count bottles.
Is it dangerous to take the recalled atorvastatin?
The issue is not likely to cause serious harm but may reduce the drug’s effectiveness. Always consult your doctor before stopping or changing medication.
What is a Class II recall?
It means the product could cause temporary or medically reversible health issues, but the risk of serious harm is low.
What should I do if I have this medicine?
Check your bottle and speak to your doctor or pharmacist to confirm if it’s affected and what steps to take next.











All this would be wonderful if it is so.
Yes totally agreed